Class 12 Chemistry Chapter 3 Electrochemistry Question Answer: Here we are providing Best Solution (Question & Answer) for class 12 Subject Chemistry 3 Electrochemistry. Students can make easily study access all the chapters of Class 12 Chemistry.
Class 12 Chemistry Chapter 3 Electrochemistry Question Answer
Multiple Choice questions-
Question 1. If the conductivity and conductance of a solution is same then its cell constant is equal to:
(a) 1
(b) 0
(c) 10
(d) 1000
Question 2. The units of conductivity are.
(a) ohm-1
(b) ohm-1 cm-1
(c) ohm-2 cm² equiv-1
(d) ohm-1 cm²
Question 3. The resistance of 0.1 N solution of acetic acid is 250 ohm, when measured in a cell of cell constant 1.15 cm-1. The equivalent conductance (in ohm-1 cm² equivalent-1) of 0.1 N acetic acid is
(a) 18.4
(b) 0.023
(c) 46
(d) 9.2
Question 4. In infinite dilution of aqueous solution of BaCl2, molar conductivity of Ba2+ and Cl– ions are = 127.32 S cm²/mol and 76.34 S cm2/mol respectively. What is A°m for BaCI2 at same dilution?
(a) 280 S cm² mol-1
(b) 330.98 S cm² mol-1
(c) 90.98 S cm² mol-1
(d) 203.6 S cm² mol-1
Question 5. The specific conductance of 0.1 M NaCl solution is 1.06 × 10-2 ohm-1 cm-1. Its molar conductance in ohm-1 cm² mol-1 is
(a) 1.06 × 10²
(b)1.06 × 10³
(c) 1.06 × 104
(d) 53
Question 6. The limiting molar conductivities A° for NaCl, KBr and KCl are 126, 152 and 150 S cm² mol-1 respectively. The A° for NaBr is
(a) 278 S cm² mol-1
(b) 976 S cm² mol-1
(c) 128 S cm² mol-1
(d) 302 S cm² mol-1
Question 7. λ(CICH2COONa) = 224 ohm-1 cm² gm eq-1, λ(NaCl) = 38.2 ohm-1 cm² gm eq-1. λ(HCl) = 203 ohm-1 cm² gm eq-1, what is the value of λ(CICH2COOH)?
(a) 288.5 ohm-1 cm² gm eq-1
(b) 289.5 ohm-1 cm² gm eq-1
(c) 388.8 ohm-1 cm² gm eq-1
(d) 59.5 ohm-1 cm² gm eq-1
Question 8. The limiting molar conductivities of HCl, CH3COONa and NaCl are respectively 425, 90 and 125 mho cm² mol-1 at 25 °C. The molar conductivity of 0.1 M CH3COOH solution is 7.8 mho cm² mol-1 at the same temperature. The degree of dissociation of 0.1 M acetic acid solution at the same temperature is
(a) 0.10
(b) 0.02
(c) 0.15
(d) 0.03
Question 9. The values of limiting ionic conductance of H and HCOO– ions are respectively 347 and 53 S cm² mol-1 at 298 K. If the molar conductance of 0.025 M methanoic acid at 298 K is 40 S cm² mol-1, the dissociation constant of methanoic acid at 298 K is
(a) 1 × 10-5
(b) 2 × 10-5
(c) 1.5 × 10-4
(d) 2.5 × 10-4
Question 10. The ionisation constant of a weak electrolyte is 2.5 × 10-5 and molar conductance of its 0.01 M solution is 19.6 S cm² mol-1. The molar conductance at infinite dilution (S cm² mol-1) is
(a) 402
(b) 392
(c) 306
(d) 39.2
Very Short Question:
Question 1. Can you store AgCl solution in Zinc pot?
Question 2. Define the term – standard electrode potential?
Question 3. What is electromotive force of a cell?
Question 4. Can an electrochemical cell act as electrolytic cell? How?
Question 5. Single electrode potential cannot be determined. Why?
Question 6. What is SHE? What is its electrode potential?
Question 7. What does the positive value of standard electrode potential indicate?
Question 8. What is an electrochemical series? How does it predict the feasibility of a certain redox reaction?
Question 9. Give some uses of electrochemical cells?
Question 10. State the factors that affect the value of electrode potential?
Short Questions:


Question 10. Depict the galvanic cell in which the reaction takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Long Questions:
Question 1. Explain construction and working of standard Hydrogen electrode? (b) Write any two differences between amorphous solids and crystalline solids.
Question 2.

Question 3.Explain how rusting of iron is envisaged as setting up of an electrochemical cell.
Question 4. Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
Question 5. Write the Nernst equation and emf of the following cells at 298 K:
Question 6. Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Assertion and Reason Questions:
1. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
- Assertion and reason both are correct statements and reason is correct explanation for assertion.
- Assertion and reason both are correct statements but reason is not correct explanation for assertion.
- Assertion is correct statement but reason is wrong statement.
- Assertion is wrong statement but reason is correct statement.
Assertion: At the end of electrolysis using platinum electrodes, an aqueous solution of copper sulphate tums colourless.
Reason: Copper in CuSO4 is converted to Cu(OH)2 during the electrolysis.
2. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
- Assertion and reason both are correct statements and reason is correct explanation for assertion.
- Assertion and reason both are correct statements but reason is not correct explanation for assertion.
- Assertion is correct statement but reason is wrong statement.
- Assertion is wrong statement but reason is correct statement.
Assertion: Zinc displaces copper from copper sulphate solution.Reason: Eº of zinc is -0.vV and that of copper is +0.34V.
Case Study Questions:
1. The concentration of potassium ions inside a biological cell is at least twenty times higher than the outside. The resulting potential difference across the cell is important in several processes such as transmission of nerve impulses and maintaining the ion balance. A simple model for such a concentration cell involving a metal M is,
M(s) | M+(aq.; 0.05 molar) || M+(aq; 1 molar) | M(s)
The following questions are multiple choice questions. Choose the most appropriate answer:
- For the above cell,
- Ecell < 0;ΔG > 0
- Ecell > 0;ΔG < 0
- Ecell < 0;ΔG° > 0
- Ecell > 0;ΔG° < 0
- If the 0.05 molar solution of M+ is replaced by a 0.0025 molar M+ solution, then the magnitude of the cell potential would be:
- 130mV
- 185mV
- 154mV
- 600mV
- The value of equilibrium constant for a feasible cell reaction is:
- < 1
- = 1
- > 1
- Zero
- What is the emf of the cell when the cell reaction attains equilibrium?
- 1
- 0
- > 1
- < 1
- The potential of an electrode change with change in:
- Concentration ofions in solution.
- Position of electrodes.
- Voltage of the cell.
- All of these.
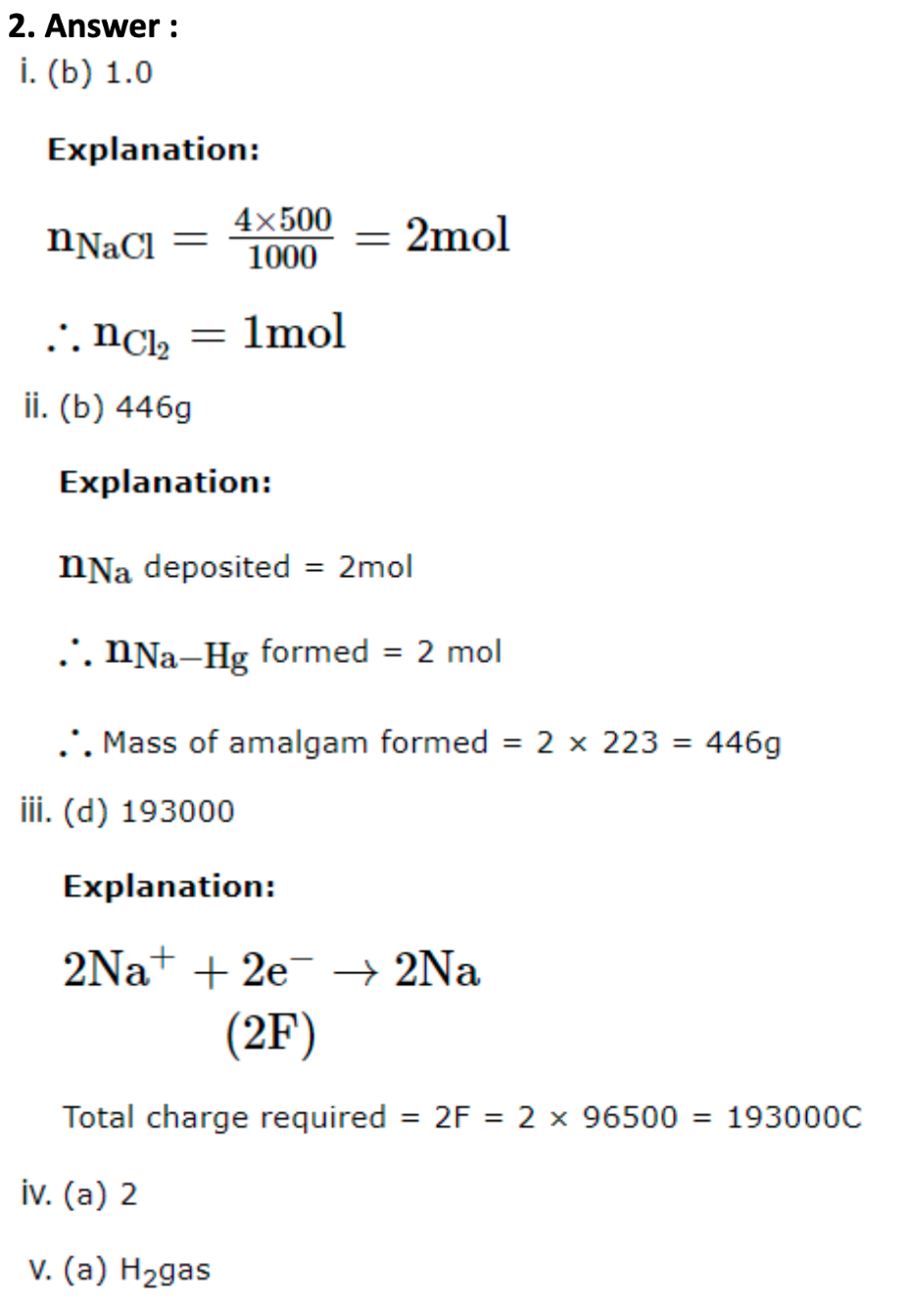
2. All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/ molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.
The following questions are multiple choice questions. Choose the most appropriate answer:
- The total number of moles of chlorine gas evolved is:
- 0.5
- 1.0
- 1.5
- 1.9
- If cathode is a Hg electrode, then the maximum weight of amalgam formed from this solution is:
- 300g
- 446g
- 396g
- 296g
- The total charge (coulomb) required for complete electrolysis is:
- 186000
- 24125
- 48296
- 193000
- In the electrolysis, the number of moles of electrons involved are:
- 2
- 1
- 3
- 4
- In electrolysis of aqueous NaCl solution when Pt electrode is taken, then which gas is liberated at cathode?
- H2gas
- Cl2gas
- O2gas
- None of these.
Answers key
MCQ answers:
- Answer: (a) 1
- Answer: (b) ohm-1 cm-1
- Answer: (c) 46
- Answer: (a) 280 S cm² mol-1
- Answer: (a) 1.06 × 10²
- Answer: (c) 128 S cm² mol-1
- Answer: (c) 388.8 ohm-1 cm² gm eq-1
- Answer: (b) 0.02
- Answer: (d) 2.5 × 10-4
- Answer: (b) 392
Very Short Answers:
- No. We can’t store AgCl solution in Zinc pot because standard electrode potential of Zinc is less than silver..
- When the concentration of all the species involved in a half-cell is unity, then the electrode potential is called standard electrode potential.
- Answer: Electromotive force of a cell is also called the cell potential. It is the difference between the electrode potentials of the cathode and anode.

- Answer: Yes, An electrochemical cell can be converted into electrolytic cell by applying an external opposite potential greater than its own electrical potential.
- Answer: A single half cell does not exist independently as reduction and oxidation occur simultaneously therefore single electrode potential cannot be measured.
- Answer: SHE stands for standard Hydrogen electrode. By convention, its electrode potential is taken as 0 (zero).
- Answer: The positive value of standard electrode potential indicates that the element gets reduced more easily than ions and its reduced form is more stable than Hydrogen gas.
- The arrangement of metals and ions in increasing order of their electrode potential values is known as electrochemical series.The reduction half reaction for which the reduction potential is lower than the other will act as anode and one with greater value will act as cathode. Reverse reaction will not occur.
- Electrochemical cells are used for determining the
- pH of solutions
- solubility product and equilibrium constant
- in potentiometric titrations
- Factors affecting electrode potential values are –
- Concentration of electrolyte
- Temperature.
Short Answers:
- Answer
The cell reaction is

Nernst Equation –

= 0.3V – 0.0394V
= +0.2606 V
- Answer:
The half-cell reactions are


= – 8488 J mol-1
- Answer:
From the reaction, n =2



- Answer:

5. Answer :

6. Answer(i)
Therefore, Required charge = 3 F
= 289461 C
(ii)

Therefore, Required charge = 2 F

= 192974 C

8. Answer:
(i) According to the question,
Now, we can write:
Electricity required for the oxidation of 1 mol of H2O to O2 = 2 F
= 192974 C
(ii) According to the question
Electricity required for the oxidation of 1 mol of FeO to Fe2O3= 1 F
= 96487 C
9. Answer :
Given,
Current = 5A
Time = 20 X 60 = 1200 s
Therefore, Charge = Current X time= 5 X 1200
= 6000 C
According to the reaction,

Nickel deposited by 2 X 96487C = 58.71 g
Therefore, nickel deposited by 6000 C

= 1.825 g
Hence, 1.825 g of nickel will be deposited at the cathode.
- Answer :
The galvanic cell in which the given reaction takes place is depicted as:

(i) Zn electrode (anode) is negatively charged.
(ii) Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
(iii) The reaction taking place at the anode is given by,

The reaction taking place at the cathode is given by,

Long Answers:
- Answer:
Construction: SHE consists of a platinum electrode coated with platinum black. The electrode is dipped in an acidic solution and pure Hydrogen gas is bubbled through it. The concentration of both the reduced and oxidized. Forms of Hydrogen is maintained at unity i.e) pressure of gas is 1 bar and concentration of Hydrogen ions in the solution is 1 molar.
Working – The reaction taking place in SHE is At 298 K , the emf of the cell constructed by taking SHE as anode and other half-cell as cathode, gives the reduction potential of the other half cell whereas for a cell constructed by taking SHE as anode gives the oxidation potential of other half cell as conventionally the electrode potential of SHE is zero.

2. Answer:

- Answer:
In the process of corrosion, due to the presence of air and moisture, oxidation takes place at a particular spot of an object made of iron. That spot behaves as the anode. The reaction at the anode is given by,
Electrons released at the anodic spot move through the metallic object and go to another spot of the object.
There, in the presence of ions, the electrons reduce oxygen. This spot behaves as the cathode. These H+ ions come either from , which are formed due to the dissolution of carbon dioxide from air into water or from the dissolution of other acidic oxides from the atmosphere in water.
The reaction corresponding at the cathode is given

The overall reaction is:

Also, ferrous ions are further oxidized by atmospheric oxygen to ferric ions. These ferric ions combine with moisture, present in the surroundings, to form hydrated ferric oxide (Fe2O3.xH20)i.e., rust.
Hence, the rusting of iron is envisaged as the setting up of an electrochemical cell.



Assertion and Reason Answers:
1. (c) Assertion is correct statement but reason is wrong statement.
Explanation:
Cu2+ ions are deposited as Cu.
2. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Case Study Answers:
1. Answer :




Class 12 Chemistry All Chapter Notes & Question Answer
- Class 12 Chemistry Chapter 1 The Solid State Notes
- Class 12 Chemistry Chapter 1 The Solid State Question Answer
- Class 12 Chemistry Chapter 2 Notes
- Class 12 Chemistry Chapter 2 Question Answer
- Class 12 Chemistry Chapter 3 Electrochemistry Notes
- Class 12 Chemistry Chapter 3 Electrochemistry Question Answer
- Class 12 Chemistry Chapter 4 Chemical Kinetics Notes
- Class 12 Chemistry Chapter 4 Chemical Kinetics Question Answer
- Class 12 Chemistry Chapter 5 Surface Chemistry Notes
- Class 12 Chemistry Chapter 5 Surface Chemistry Question Answer
- Class 12 Chemistry Chapter 6 Isolation of Elements Notes
- Class 12 Chemistry Chapter 6 Isolation of Elements Question Answer
- Class 12 Chemistry Chapter 7 The p-Block Elements Notes
- Class 12 Chemistry Chapter 7 The p-Block Elements Question Answer
- Class 12 Chemistry Chapter 8 The D And F Block Elements Notes
- Class 12 Chemistry Chapter 8 The D And F Block Elements Question Answer
- Class 12 Chemistry Chapter 9 Coordination Compounds Notes
- Class 12 Chemistry Chapter 9 Coordination Compounds Question Answer
- Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes Notes
- Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes Question Answer
- Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers Notes
- Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers Question Answer
- Class 12 Chemistry Chapter 12 Aldehydes Ketones and Carboxylic Acids Notes
- Class 12 Chemistry Chapter 12 Aldehydes Ketones and Carboxylic Acids Question Answer
- Class 12 Chemistry Chapter 13 Amines Notes
- Class 12 Chemistry Chapter 13 Amines Question Answer
- Class 12 Chemistry Chapter 14 Biomolecules Notes
- Class 12 Chemistry Chapter 14 Biomolecules Question Answer
- Class 12 Chemistry Chapter 15 Polymers Notes
- Class 12 Chemistry Chapter 15 Polymers Question Answer
- Class 12 Chemistry Chapter 16 Chemistry in Everyday Life Notes
- Class 12 Chemistry Chapter 16 Chemistry in Everyday Life Question Answer