Class 11 Biology Chapter 9 Biomolecules Notes and Question Answer: Very well-prepared notes for chapter 9 “Biomolecules” by subject experts will help you in all types of exams. Along with this, where important questions and their answers and question bank have been given, which you can prepare according to your exam.
Class 11 Biology Chapter 9 Biomolecules Notes and Question Answer
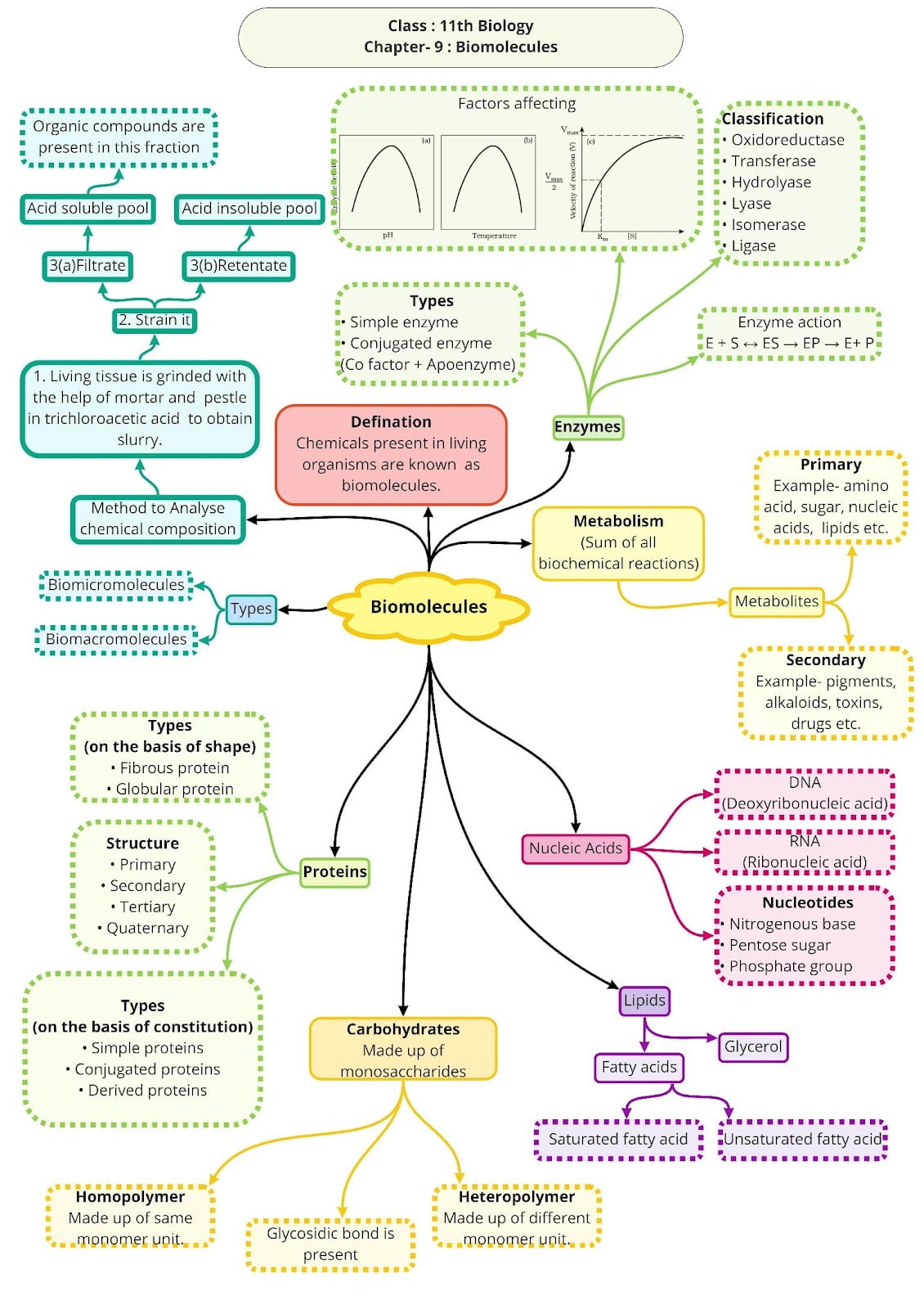
Biomolecules: All the carbon compounds that we get from living tissues.

Biomacromolecules: Molecules which have molecular weights less than one thousand Dalton. They are also known as monomers.

Biomacromolecules: Have molecular weight more than 10000 Daltons (generally 10,000 Daltons and above). They are generally polymers. A biomolecule a with molecular weight in the range of ten thousand daltons and above; found in acid insoluble fraction. e.g. polysaccharides, nucleic acids, proteins and lipids.

Nucleic acids
Primary and secondary metabolites
- Primary metabolites: have identifiable functions and play important roles in normal physiological process eg. Amino acids, nitrogenous bases, proteins and nucleic acid.
- Secondary metabolites: are product of certain metabolic pathways from primary metabolites, eg. carotenoids, drugs, alkaloids, essential oils, rubber, gum, cellulose and resins etc.
Amino acids
Organic compounds containing an amino group and one carboxyl group (acid group) and both these groups are attached to the same carbon atom called α carbon and so they are called amino acids.

e.g. In Glycine R = H
In alanine R = CH3
In serine R = CH2 – OH
Twenty types of amino acids. Amino acid exists in Zwitterionic form at different pHs.

Based on number of amino and carboxyl groups, amino acids can be:
Aromatic: Tryptophan, phenylalanine and Tyrosine are aromatic (give smell) amino acids.

Proteins: Proteins are polypeptide chains made up of amino acids. There are 20 types of amino acids joined together by peptide bond between amino and carboxylic group. There are two kinds of amino acids.
Essential amino acids are obtained by living organism along with food.

Non-essential amino acids can be prepared by our body from raw materials.

Biological macromolecules
There are three main types of biological macromolecules, according to mammalian systems:
- Carbohydrates
- Nucleic acids
- Proteins
- Lipids
Carbohydrates: Carbohydrates are polymers of carbon, hydrogen and oxygen. They can be classified as monosaccharides, disaccharides and polysaccharides. Carbohydrates are found in starch, fruits, vegetables, milk and sugars. They are an important source of a healthy diet.

Nucleic Acids: The nucleic acids include DNA and RNA that are the polymers of nucleotides. Nucleotides comprise a pentose group, a phosphate group, and a nitrogenous base group. All the hereditary information is stored in the DNA. The DNA synthesized into RNA and proteins.
Proteins: Proteins are the polymers of amino acids. These include the carboxylic and the amino group. There would be no lipids or carbohydrates without proteins because the enzymes used for their synthesis are proteins themselves.
Lipids: Lipids are a hydrophobic set of macromolecules, i.e., they do not dissolve in water. These involve triglycerides, carotenoids, phospholipids, and steroids. They help in the formation of the cell membrane, formation of hormones and in the and as stored fuel.
Fatty Acids & Saturated
With single bonds in carbon chain, e.g., Palmitic acid, butyric acid.
Unsaturated
With one or more double bonds, e.g., oleic acid, linoleic acid.
Glycerol
A simple lipid, is trihydroxy propane.

Some lipid have fatty acids esterified with glycerol. Example of fatty acid (Palmitic acid)
(CH3 — (CH2)14 — COOH)

Triglyceride (R1, R2, R3 are alkyl groups in fatty acids.)
Nitrogen bases (Carbon compounds with heterocyclic rings)
Purine: Adenine, Guanine,

Pyrimidine: Cytosine, Uracil, Thymine.

Nucleoside: Nitrogenous base + Sugar e.g., Adenosine, guanosine.

Nucleotide: Nitrogenous base + Sugar + Phosphate group. e.g. Adenylic acid, Guanylic acid. Thymidylic acid.
Nucleic acids: Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
Examples of Macromolecules:
Synthetic Fibres

- Nylon, rayon and spandex consist entirely of macromolecules. These are created in certain steps:
- The monomers are reacted to make prepolymers or a liquid, primitive macromolecule. In the next step, the prepolymers are fed through a cell where it solidifies and attains the desired thickness. This process is called spinning.
Genetic Transfer: DNA is a genetic material that contains nucleic acids which code for genetic material. During meiosis, the DNA is no longer a whole, and the nucleotides that remain are responsible for transferring the genetic information to the gametes.
Monomers and Polymers
Macromolecules are basically polymers, long chains of molecular sub-units called monomers. Carbohydrates, proteins and nucleic acids are found as long polymers. Due to their polymeric nature and large size, they are known as macromolecules.
Structure of Proteins
- Primary structure: Is found in the form of linear sequence of amino acids. First amino acid is called N-terminal amino acid and last amino acid is called C-terminal amino acid.
- Secondary structure: Polypeptide chain undergoes folding or coiling which is stabilized-by hydrogen bonding. Right handed helices are observed; e.g., fibrous protein in hair, nails.
- Tertiary structure: Long protein chain is folded upon itself like a hollow woollen ball. Gives a 3-dimensional view of protein, e.g., myosin.
- Quaternary structure: Two or more polypeptides with their folding’s and coiling’s are arranged with respect to each other, e.g., Human haemoglobin molecule has 4 peptide chains – 2 α and 2 β Subunits.
Monosaccharides
Monosaccharides are joined by glycosidic bond, right end is reducing and left end is non reducing.
Polysaccharides
Are long chain of polymers of monosaccharides.
- Starch: Store house of energy in plant tissues. Forms helical secondary structures, made of only glucose monomers.
- Cellulose: Homopolymer of glucose. It does not certain complex helices. Cotton fibre is cellulose.
- Glycogen: Is a branched homopolymer, found as storage polysaccharide in animals.
- Insulin: Is a polymer of fructose.
- Chitin: Chemically modified sugar (amino-sugars) N-acetyl galactosamine form exoskeleton of arthropods; heterpolymer.

Metabolic Pathways
There are two types of metabolic pathways:
- Anabolic pathways: Lead to formation of more complex structure from a simpler structure with the consumption of energy, e.g., Protein from amino acids., also known as biosynthetic pathways.
- Catabolic pathway: Lead to formation of simpler structure from a complex structure, e.g., Glucose → Lactic Acid + energy The most important energy currency in living systems is ATP (adenosine tri – phosphate).

Bonds linking monomers in a polymer
Peptide bond: Formed between the carboxyl (–COOH) group of one amino acid, and the amino (– NH2) group of the next amino with the elimination of water moiety, (dehydration).
Glycosidic bond: Bond formed between two carbon atoms of two adjacent monosaccharides., by dehydration.
Phosphodiester bond: Bond formed in nucleic acids where in a phosphate moiety links the 3-carbon of one sugar of one nucleotide to the 5-carbon of the sugar of the succeeding nucleotide. (The bond between phosphate group and hydroxyl group of sugar)
Enzymes
- Enzymes are commonly proteinaceous substances which are capable of catalysing chemical reactions of biological origin without themselves undergoing any change. They are commonly called as biocatalysts.
- The nucleic acids that behave like enzymes are called ribozymes.
- The tertiary structure of protein/Enzyme has pockets or crevice into which substrate fit to form ES complex.
- The formation of the ES complex is essential for catalysis. E + S = ES → EP → E + P
- The structure of substrate gets transformed into the structure of product through formation of transient state structure.
- The major difference between inorganic and organic catalyst is inorganic catalyst works effectively at high temperature and pressure but enzyme get damaged at high temperature.
- The external energy required to start a chemical reaction is called activation energy.

Factors influencing Enzyme Activity:
- Temperature: An enzyme is active within a narrow range of temperature. Temperature ate which enzyme is most active is called optimum temperature. The enzyme activity decrease above and below this temperature.
- pH: Every enzyme has an optimum pH at which it is maximum active. Most of the intracellular enzymes work at neutral pH.
- Concentration of Substrate: Increase in substrate concentration increases the rate of reaction due to occupation of more active sites by substrate.
- Competitive Inhibitor: When the molecular structure of inhibitor resembles the substrate, it inhibits the function of enzymes.

Enzymes are classified as:
- Oxidoreductases/ Dehydrogenases: S reduced + S’ oxidized S oxidized + S’ reduced
- Transferases: S – G + S’ S + S’ – G
- Hydrolases: Catalyses the hydrolysis of peptide, ester, glycosidic bonds et
- Lyases: Remove the groups from substrate.
- Isomerases: Inter conversion of optical, geometrical or positional isomers.
- Ligases: Catalyses the linking together of two compounds.
Co-factors
Co-factors are the non-protein constituent of an enzyme which make the enzyme more catalytically active. The protein portions of enzyme are called apoenzyme.
- Prosthetic group: These are organic compound which tightly bound to the apoenzyme.
e. g., Haem is prosthetic group in peroxidase and catalase.
- Coenzyme: These are organic compounds whose association with the apoenzyme is only transient, usually occurring during the course of catalysis.
e.g., Coenzyme Nicotinamide adenine dinucleotide (NAD) and NADP contain vitamin niacin.
- Metal ions: Metal ions form coordination bond with side chains at the active site and at the same time form one or more coordination bond with substrate.
e.g., zinc in enzyme carboxy peptidase.

Class 11 Biology Chapter 9 Biomolecules Mind Map
Class 11 Biology Chapter 9 Biomolecules Question Answer
Important Questions
- Multiple Choice Questions:
Question 1. Glucose is a
(a) Ketose hexose sugar
(b) Pyronose pentose sugar
(c) Aldose hexose sugar
(d) Furanose pentose sugar.
Question 2. Lactose molecule is composed of
(a) Fructose+Fructose
(b) Glucose+Fructose
(c) Glucose+Glucose
(d) Glucose+Galactose.
Question 3. Which group contains all polysaccharides?
(a) Glycogen, sucrose and maltose
(b) Maltose, lactose and sucrose
(c) Glycogen, glucose and sucrose
(d) Glycogen, cellulose and starch.
Question 4. Amino acids are formed from
(a) Proteins
(b) Fatty acids
(c) Volatile acid
(d) α < -keto acids.
Question 5. A nucleoside is formed of
(a) Phosphate and nitrogen base
(b) Pentose sugar and phosphate
(c) Pentose sugar, phosphate and nitrogen base
(d) Pentose sugar and nitrogen base.
Question 6. The most abundant component of a cell is
(a) Lipid
(b) Protein
(c) Water
(d) Cellulose
Question 7. Maximum amount of iron occures in
(a) Proteins
(b) Bone cells
(c) Leucocytes
(d) Erythrocytes.
Question 8. Calcium is required for
(a) Blood clotting
(b) Bone formation
(c) Muscle contraction
(d) All of these.
Question 9. Immediate source of energy is
(a) ATP
(b) Glucose
(c) NADH
(d) Pyruvic acid.
Question 10. An amino acid without an asymetrical carbon atoms
(a) Glycine
(b) Threonine.
(c) Proline
(d) Histidine.
Question 11. The most abundant protein is
(a) Glycine
(b) Valine
(c) Arginine
(d) Collagen
Question 12. Basic unit of nucleic acid is
(a) Pentose sugar
(b) Nucleotide
(c) Phosphoric acid
(d) Nitrogen base.
Question 13. The amino acids which are not synthesized in our body are called
(a) Deaminated
(b) Non-essential
(c) Essential
(d) Proteinaceous.
Question 14. Which of the following is a non-reducing sugar?
(a) Lactose
(b) Glucose
(c) Maltose
(d) Sucrose.
Question 15. The primary structure of a protein is due to
(a) ionic bonds
(b) hydrogen
(c) Peptide bonds
(d) S-S linkage
- Fill In the Blanks:
- All the elements present in a sample of ………….. are also present in a sample of living tissue.
- One is called the filtrate or more technically, the acid soluble, peol, and the second, the retentate or the acid insoluble ………..
- One ………… and ………… a compound.
- Amino acids are ……………… containing an amino group and an acidic group as substituents on the some carbon i.e., the α < -carbon
- The …………… and ………… properties of amino acids are essentially of the amino, carboxyl and the ‘R’ functional groups.
- ………… are generally water insoluble.
- True or False:
- Biomacromolecules are polymers. They are made of building blocks which are different.
- Proteins are heteropolymers made of starch acids.
- Nucleic acids (RNA and DNA) are composed of nucleotides.
- Enzyme, are composed of one or several polypeptide chains.
- When the binding of the chemical shuts off enzyme activity, the process is called inhibition and the chemical is called an inhibitor.
- When the inhibitor closely resembles the substrate in its mo-lecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor.
- Very Short Question:
- What is hydrolysis?
- Define fatty acid.
- What are iso-enzymes?
- Give the names of 2 non-polar organic solvents that are used for lipid extraction from cells.
- Name one monosaccharide sugar that is found in the blood plasma of human beings.
- What is the function of calcium in the human body?
- Name the bonds uniting the monosaccharide subunits.
- What are lygases?
- Name the ending group of fatty acids which are organic acids with a hydrocarbon chain.
- Which is the most common form of sugar in fruits?
- Short Questions:
- What are monosaccharides? Give few examples.
- What is a disaccharide?
- Why do fats release more energy than carbohydrates on oxidation?
- What is the function of calcium in our body? In what form is calcium deposited in the middle lamella?
- Define cellular pool. What are the characteristics of a small molecule in the cellular pool?
- What are lipids or fats? State their characteristic. What are the functions of subcutaneous fat in our body?
- How are amino acids linked to form a peptide chain?
- What are phospholipids?
- Long Questions:
- Enlist the functions of small carbohydrates?
- Enumerate the functions of Lipids.
- How does water help in maintaining the constancy of the internal environment of an organism?
- What are peroxisomes and phagosomes?
- Enumerate the importance of Energy carriers.
Assertion Reason Question-
- In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
(c) If Assertion is true but Reason is false.
(d) If both Assertion and Reason are false.
Assertion: The living state is a equilibrium steady state to be able to perform work.
Reason: Living process is a constant effort to prevent falling into non-equilibrium.
- In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
(c) If Assertion is true but Reason is false.
(d) If both Assertion and Reason are false.
Assertion: Living organisms have more nitrogen and oxygen per unit mass than inanimate objects (e.g., earth crust).
Reason: Living organisms have more Ca, Mg, Na in them than inanimate object.
Case Study Based Question-
- Identify the molecular structure of the given polysaccharide and answer the questions that follow

(i) Chooose the correct characteristics about the polysaccharide molecule shown above.
I. It is a storage polysaccharide in animal cells only.
II. It gives red colour on reaction with iodine.
III. It is a branched homopolysaccharide.
IV. It contains repeating units of fructose only.
(a) I and IV
(b) II, III and IV
(c) Only III
(d) I, II and III
(ii) All the listed polysaccharides are homopolymers except
(a) starch
(b) glycogen
(c) chitin
(d) cellulose
(iii) Inulin is a polymer of
(a) glucose
(b) fructose
(c) glucose + sucrose
(d) fructose + galactans
(iv) A polysaccharide found in the exoskeleton of crabs is
(a) cellulose
(b) pectin
(c) murein
(d) chitin
(v) Murein is a heteropolysaccharide like
(a) araban
(b) xylan
(c) hyaluronic acid
(d) agar
- Identify the molecular structure of lipid molecule and answer the questions that follow

(i) Choose the incorrect characteristic about the lipid molecule shown above
I. It is a common sterol molecule.
II. It is the precursor of steroid hormones.
III. The given molecule has a steroid nucleus, a hydrocarbon side chain, and a hydroxyl group.
IV. Cholesterol is soluble in blood and thus transport through bloodstream easily.
(a) Only IV
(b) III and IV
(c) I and II
(d) Only II
(ii) Lipids mainly consist of
(a) carbon only
(b) carbon, hydrogen and nitrogen
(c) carbon, hydrogen and oxygen
(d) hydrogen only
(iii) Saturated fatty acids contain
(a) double bond
(b) carboxyl group
(c) Both (a) and (b)
(d) None of the above
(iv) An example of unsaturated fatty acid is
(a) oleic acid
(b) stearic acid
(c) linoleic acid
(d) Both (a) and (c)
(v) Among the given options, non-polymeric molecule is
(a) nucleic acid
(b) proteins
(c) lipids
(d) polysaccharide
- Answer Key-
- Multiple Choice Answers:
- (c) Aldose hexose sugar.
- (d) Glucose+ Galactose.
- (d) Glycogen, cellulose and starch.
- (d) α < -keto acids.
- (d) Pentose sugar and nitrogen base.
- (c) Water.
- (d) Erythrocytes.
- (d) All of these
- (a) ATP.
- (a) Glycine.
- (d) Collagen.
- (b) Nucleotide.
- (c) Essential.
- (d) Sucrose.
- (c) Peptide bonds.
- Fill In the Blanks:
- earth’s crust
- fraction.
- isolates, purifies
- organic compounds
- chemical, physical
- Lipids
- True or False:
- True
- False
- True
- True
- True
- True
- Very Short Answers:
- Answer: During the digestion of carbohydrates, the glycosidic bond between sugar residues is broken by the addition of water and this is called hydrolysis.
- Answer: Fatty acids are organic acids with a hydrocarbon chain ending in a carboxyl group.
- Answer: The enzymes possessing slightly different molecular structures but similar in their bio-catalytic action.
- Answer: Chloroform, Ether.
- Answer: Glucose.
- Answer: Calcium in bones and teeth provides strength and rigidity to them.
- Answer: Monosaccharide sub-units are joined together by glycoside bonds.
- Answer: Lygases are the enzymes that join two substrate molecules.
- Answer: Carboxyl.
- Answer: Fructose.
- Short Answer:
- Answer: Monosaccharides are the simplest carbohydrates that cannot be hydrolyzed into still smaller carbohydrates. The general formula is Cn H2n On e.g. Ribose, Glucose, Fructose.
- Answer: A disaccharide is a sugar molecule composed of two monosaccharide sub-units e.g. a molecule of sucrose is formed from a molecule of glucose and a molecule of fructose by dehydration.

- Answer: Like carbohydrates, fats are made up of C, H, and O but they contain fewer oxygen molecules than carbohydrates. On oxidation they consume more oxygen releasing more energy.
- Answer: Calcium is impregnated in bones and teeth. It provides them with strength and rigidity.
Calcium is deposited in the middle lamella in the form of calcium pectate.
- Answer: The collection of various types of molecules in a cell is termed a cellular pool.
The characteristics of small molecules in the cellular pool are
- Low molecular weight
- Simple molecular conformation.
- Higher solubility.
- Answer: Fat or lipids are esters of glycerol and fatty acids. They are made up of C, H, and O but include proportionately less oxygen as compared to carbohydrates. They are insoluble in water and soluble in non-polar organic solvents.
Functions of subcutaneous fat are
Storage of food (chemical form of energy).
Shock absorption.
Insulation.
- Answer: Amino acids are condensed together to form a peptide chain. The bond is formed between the carboxyl group of one amino acid and the amino group of adjacent amino acid. This is called a peptide bond and it is formed by dehydration.
- Answer: Phospholipids are lipids containing phosphate groups e.g. phosphoglyceride. They have a hydrophilic polar head and a hydrophobic non-polar tail.

- Long Answer:
- Answer:
i. Monosaccharides are formed during the photosynthetic pathway. They are stored in plants and are utilized by other living organisms depending on them.
ii. Glucose is the blood sugar of many animals and on oxidation, it provides energy for all vital activities.
iii. Nucleotides and nucleosides contain pentose sugar in the form of ribose and deoxyribose sugars. They form a part of nucleic acids.
iv. Lactose of milk is formed from glucose and galactose and mammary glands of mammals.
v. Glucose is used for the synthesis of fats and amino acids.
vi. Structural polysaccharides like cellulose and oligosaccharides are derived from mono-saccharides.
vii. Food storage polysaccharides like starch and glycogen are derived from monosaccharides.
- Answer:
1. Lipids are storage products in plants as well as animals.
(a) In plants, fats are stored in cotyledons or endosperm to provide nourishment to the developing embryo.
(b) In animals fats are stored in adipocytes to be used whenever required by the body.
2. In animals, subcutaneous fats act as an insulation layer and shock \ absorber.
3. They form structural components of membranes, phospholipids, glycolipids, and sterols.
4. They take part in the synthesis of steroid hormones, vitamin D, and bile salts.
5. Act as a solvent for fat-soluble vitamins i.e., vitamin A, D, E, and K.
6. The neutral fats form a concentrated fuel producing more than twice as much energy per gram as do the carbohydrates. They thus, represent an economical food reserve in the body.
7. The wax lipids form a waterproof protective coating on animal furs, plant stem, leaves, and fruits.
- Answer: Some substances, capable of neutralizing acids or bases, remain in solution in the cytoplasm as extracellular fluids, e.g., bicarbonate (HCO3), carbonic acid, dibasic phosphate (HPO4-2). Acids and bases mix in the body fluids with these substances and are neutralized by them. Because of its solvent action water aids in keeping a constant pH.
Water also helps in maintaining constant body temperature by eliminating excess heat through the evaporation of sweat. Elimination of waste products through urine also helps in maintaining the constancy of the internal environment of an organism.
- Answer: Peroxisomes: These were for the first time observed in the kidney of rodents. They are found both in plants and animals. Their size varies from 0.5 to lp in diameter. They are delimited by a single membrane and contain a finely granular matrix. They often possess a central core called nucleoid which may consist of parallel tubules or twisted with strands. Peroxisomes are generally observed in close association with the endoplas¬mic reticulum.
Peroxisomes in different plant and animal cells differ con¬siderably in their enzymatic make-up, but they contain some peroxide-producing enzymes like urate, oxidase, D-amino acid oxidase, B-hydroxy acid oxidase, and catalase. Peroxisomes are somehow associated with some metabolic processes like photorespiration and lipid metabolism in animal cells.
Sphaerosomes: There are cell organelles bounded by a single membrane. They contain enzymes and are visible under the light microscope. These show some affinities for fat stains, including Sudan stain and sodium tetroxide.
These organelles originate from E.R. by budding. They contain enzymatic proteins which help in synthesizing oils and fats. Further devel¬opment of phagosomes takes place through an increase in the lipid content with a concomitant decrease in protein.
- Answer: Energy carriers consist of nucleotides having one or two additional phosphate groups linked up at their phosphate end forming diphosphates and triphosphates. Linkage of additional phosphate groups occurs at the cost of a large amount of energy. This energy is provided by the oxidation of food mainly glucose and by photosynthesis.
Separation of the additional phosphate groups from the nucleotides by enzymatic hydrolysis releases a correspondingly large amount of energy.
Thus, ADP and ATP provide ready energy for biological activities.
The bonds joining the additional phosphate groups to the nucleotides are called high energy or energy-rich bonds, as they carry a great deal of energy. The nucleotides having more than one phosphate group are called higher nucleotides.
The energy of energy carriers, when set free is utilized for driving energy-dependent reactions in the cell and is biologically useful energy. ATP is the most common energy carrier in cells and is often called the energy currency of the cell.
Assertion Reason Answer-
- (d) If both Assertion and Reason are false.
Explanation: The living systems are in metabolic flux and thus, maintain the concentration of biomolecules, always remaining in nonequilibrium steady state where equilibrium is seldom achieved. No work can be carried out in equilibrium state. Living systems are therefore, regularly receiving an input of energy to prevent reaching an equilibrium and always remain in non-equilibrium steady state. Energy is obtained from metabolism. Metabolism and living state are thus, complementary and synonymous.
- (c) If Assertion is true but Reason is false.
Explanation: After performing elemental analysis of a plant tissue, animal tissue, microbial paste (living matter) and of a piece of earth’s crust (animate object), it was found that all living and non-living systems are made up of same chemical i.e., elements (e.g. carbon, hydrogen, oxygen and several others). Most living organisms have relatively high abundance of carbon and hydrogen than in earth’s crust.
Case Study Question-
- Answer:
(i) (d)
(ii) (c)
(iii) (b)
(iv) (d)
(v) (c)
- Answer:
(i) (a)
(ii) (c)
(iii) (b)
(iv) (d)
(v) (c)
Class 11 Biology All Chapter Notes and Question Answer
- Class 11 Biology Chapter 1 The Living World Notes and Question Answer
- Class 11 Biology Chapter 2 Biological Classification Notes and Question Answer
- Class 11 Biology Chapter 3 Plant Kingdom Notes and Question Answer
- Class 11 Biology Chapter 4 Animal Kingdom Notes and Question Answer
- Class 11 Biology Chapter 5 Morphology of Flowering Plants Notes and Question Answer
- Class 11 Biology Chapter 6 Anatomy of Flowering Plants Notes and Question Answer
- Class 11 Biology Chapter 7 Structural Organisation In Animals Notes and Question Answer
- Class 11 Biology Chapter 8 Cell The Unit Of Life Notes and Question Answer
- Class 11 Biology Chapter 9 Biomolecules Notes and Question Answer